How long will it take to freeze dry my samples?

People in the lab often contact us with the seemingly germane question, “How long will it take to freeze dry my samples?” We wish we could give a definitive answer to this question, however the answer we have to give is: It depends. Given the numerous potential applications and conditions, the length of the freeze dry process will vary.
The majority of laboratory samples will lyophilize in 24 to 48 hours but there are certainly exceptions.
The freeze drying process is dependent on a variety of factors that determine the amount of time the freeze drying process requires.
Sample type
The freezing temperature (eutectic point) of the sample and the presence of salts, sugars, or solvents will influence the length of time required to lyophilize successfully. A low freezing point sample or one that is susceptible to melt back, will require a longer lyophilization cycle.
Sample size
The more volume to lyophilize, the longer the freeze dry run.
Flask size
Spreading a sample out over a greater surface area allows more contact for heat input and shortens the distance in which the drying front has to move through the sample. As a general rule, freeze dry flasks should be filled to only one-third of their total volume.
How the sample was frozen
Faster pre-freezing rates, result in the formation of small ice crystals. Small ice crystals generally require longer freeze drying runs. When samples have small ice crystals, the cake that is formed in subl
imation, is less porous and harder for water molecules to move through. This slows the sublimation rate.

Freeze dry system
It is important to select the correct freeze dryer for the sample. The freeze dryer’s collector should be 15°C-20°C colder than the sample’s freezing point. The instantaneous load capacity and the collector capacity should be able to accommodate the sample volume. If either one of these parameters are undersized, the rate of sublimation will be affected.
Amount of heat and how heat is transferred to the sample
Energy in the form of heat is what is driving the lyophilization process. The more heat that the sample gets, the faster the run.
Because freeze drying is such a popular laboratory method, there are thousands of methods published on a wide variety of sample types. Before you begin, search online for published methods for your type of sample or as close to your sample type as you can find. Use the published parameters as a starting point.
When developing a lyophilization protocol the first objective should be to first; freeze dry successfully, then optimize the run time. We recommend beginning your method development with non-valuable samples that are similar to your real sample, as it may take several trials to get the parameters correct.
Once a working protocol is established that produces consistent results, then freeze dry the more valuable samples. After you have established a method that is producing good results, the next step is to tweak the parameters to reduce the run time.
To learn more about optimizing lyophilization parameters, read the article, How to Freeze Dry Faster.
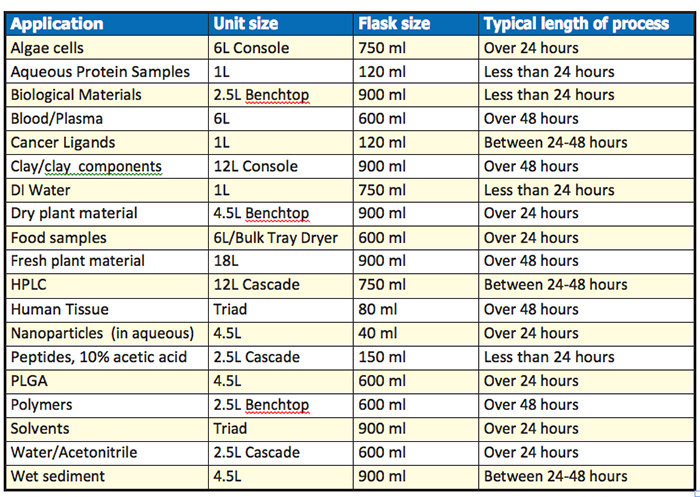
Here are some examples of applications and their typical run times from FreeZone customers.
| chevron_left | 4 Reasons you shouldn't dry evidence in a fume hood | Articles | 4 Reasons You Should Use a Remote Blower Instead of a Built-In Blower | chevron_right |






